Research Summary:
Our research seeks to develop new methods for the synthesis of functional polymers with the goal of discovering and studying their emergent macromolecular behavior. The approach is rooted in the belief that the convergence of organic, continuous-flow, and polymer chemistries holds the key to making materials smarter, more functional, and more sustainable. The group is currently focused on developing methods to control stereochemistry in ionic polymerizations, uncovering enhanced function in commodity polymers through selective C–H functionalization, removing harmful ‘forever chemicals’ from water, and creating automated approaches for the synthesis of unique polymer architectures with novel functions.
Our research program spans each stage from molecular design to material function and will provide students with a diverse and competitive skill set bridging organic and polymer synthesis, small molecule and macromolecule characterization, and applied studies in material science and biotechnology. The goals of the research are inherently interdisciplinary and students will routinely work collaboratively both within and outside of the group to accomplish their scientific and professional goals. We envision our research efforts providing new and potentially useful solutions to challenges in sustainability and human health.
Research Areas of Interest:
Stereoselective Polymerizations
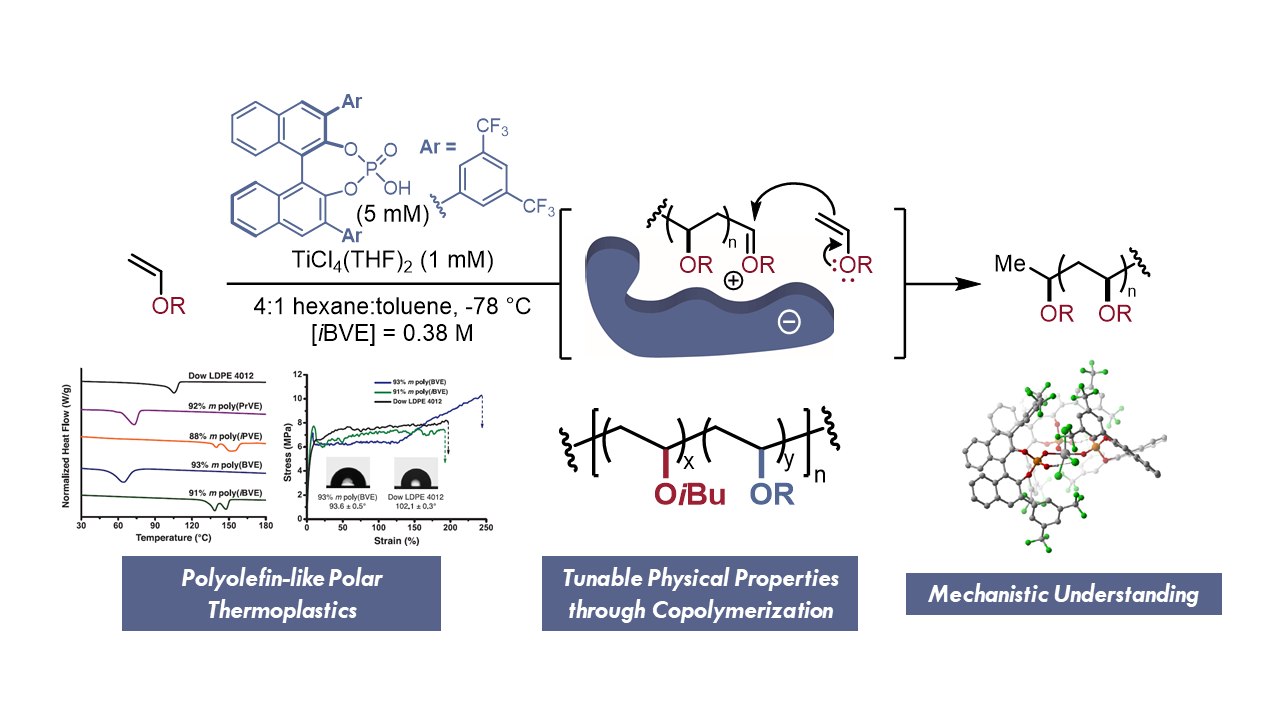
The tacticity of polymers can significantly affect their thermomechanical properties. In our lab, we are interested in identifying new platform mechanistic approaches to control configurational (i.e., tacticity) and conformational (i.e., helicity) stereochemistry. Early on, we recognized the potential of asymmetric ion pairing catalysis to control stereochemistry in cationic polymerization. We have applied this concept to the stereoselective polymerization of vinyl ether monomers and the helix-sense selective polymerization of N-vinyl carbazoles. The new materials discovered through these approaches have been explored as sustainable thermoplastics, chiro-optictal materials, and spin-transport materials.
Our interests in stereoselective polymerization have more recently expanded beyond asymmetric ion pairing catalysis. We recognized the potential of stereoconvergent polymerization to provide stereodefined materials from chiral, racemic building blocks. Our report of the first stereoconvergent polymerization provides a conceptual framework to efficiently access a broad range of new polymers through novel mechanistic approaches.
Representative Publications:
J. R. Jagannathan & F. A. Leibfarth, Stereoconvergent Chain Growth Polymerization. ACS Central Science, 2025, 11, 797-804. (doi)
H. Yeo, C. C. Sorensen, H. Tahir, A. Marquardt, Y. F. Yang, N. Legaux, B. M. Savoie, F. A. Leibfarth, & B. W. Boudouris. Stereoregular Radical Polymers Enable Selective Spin Transfer. Science Advances 2025, 11, eadr4004. (doi)
C. C. Sorensen & F. A. Leibfarth. Stereoselective Helix-Sense-Selective Cationic Polymerization of N-vinylcarbazole using Chiral Lewis acid Catalysis. J. Am. Chem. Soc. 2022, 144, 8487-8492. [doi]
T. P. Varner, A. J. Teator, Y. Reddi, P. E. Jacky, C. J. Cramer & F. A. Leibfarth. Mechanistic Insight into the Stereoselective Polymerization of Vinyl Ethers. J. Am. Chem. Soc. 2020, 142, 17175-17186. (doi)
A. J. Teator, F. A. Leibfarth*. Catalyst-Controlled Stereoselective Cationic Polymerization of Vinyl Ethers. Science, 2019, 363, 1439-1443. (doi)
Polymer C–H Functionalization
C–H functionalization allows for the modification of material properties with the potential to increase the value of polymers and provide pathways to more sustainable thermoplastics. In our lab, our efforts have been focused on the C–H functionalization of polyolefins using amidyl radical reactive intermediates. The reagents that we design, in close collaboration with the Alexanian Group, provide a diverse array of functionalization to polyolefins without the chain scission or uncontrolled branching that limits other radical-based techniques. We focus on developing methods that work without solvent in an extruder, which allows the chemistry to be conducted in widely available polymer processing infrastructure. We have applied these approaches to the synthesis of polyolefin ionomers, dynamic polyolefin networks, and structural adhesives
Representative Publications:
E. K. Neidhart, M. Hua, Z. Peng, L. T. Kearney, V. Bhat, F. Vashahi, E. J. Alexanian, S. S. Sheiko, C. Wang, B. A. Helms* and F. A. Leibfarth*. C–H Functionalization of Polyolefins to Access Reprocessable Polyolefin Thermosets. J. Am. Chem. Soc. 2023, 145, 27450-27458. [doi]
C. Jehanno, J. W. Alty, S. De Meester, A. P. Dove, E. Y. -X. Chen, F. A. Leibfarth & H. Sardon. Upcycling Commodity Polymers: Critical Advances and Future Opportunities. Nature 2021, 603, 803-814. (doi)
T. J. Fazekas, J. W. Alty, A. S. Miller, E. K. Neidhart, F. A. Leibfarth & E. J. Alexanian. A General Strategy for the Diversification of Aliphatic C–H Bonds via Radical Chain Transfer. Science 2021, 375, 5445-550. [doi]
S. E. Lewis, B. E. Wilhelmy Jr. & F. A. Leibfarth*. Upcycling Aromatic Polymers through C–H Functionalization. Chem. Sci., 2019, 10, 6270-6277. (doi)
J. B. Williamson; W. L. Czaplyski; E. J. Alexanian* & F. A. Leibfarth*. Regioselective C–H Xanthylation as a Platform for Polyolefin Functionalization. Angew. Chem. Int. Ed. 57, 6261-6265. (doi)
Interfacing automated polymerization and data science
We develop automated, flow chemistry approaches for the synthesis of material libraries. Flow Chemistry is a technique that is highly reproducible, allows for rapid mixing and efficient heat transfer, and high sample throughput. Our work has advanced automated approaches for both chain- and step-gorwth polymerizations, which has enabled the high throughput synthesis of polymer libraries for applications where multiple performance criteria must be optimized simultaneously, including 3D printing resins and 19F MRI copolymers. Working with the Isayev group, we interface these synthetic technologies with modern data science approaches, including active and reinforcement learning, to identify structure–property relationships and discovery high performing materials.
Representative Publications:
J. L. Rapp, D. M. Anstine, F. Gusev, F. Nikitin, K. Yun, M. Borden, V. Bhat, O. Isayev* & F. A. Leibfarth*. Design of Tough 3D Printable Elastomers with Human-in-the-loop Reinforcement Learning. Angew. Chem. Int. Ed. 2025, e202513147. [doi]
M. Reis, F. Gusov, N. G. Taylor, S. H. Chung, Y. Z. Lee, O. Isayev* & F. A. Leibfarth*. Machine Learning-Guided Discovery of 19F MRI Agents Enabled by Automated Copolymer Synthesis. J. Am. Chem. Soc. 2021, 143, 17677-17689. [doi]
M. H. Reis, T. P. Varner, & F. A. Leibfarth*. The Influence of Residence Time Distributions on Continuous-flow Polymerization. Macromolecules 2019, 52, 3551-3557. (doi)
M. H. Reis, C. L. G. Davidson IV & F. A. Leibfarth*. Continuous-flow Chemistry for the Determination of Comonomer Reactivity Ratios. Polym. Chem. 2018, 9, 1728-1734. (doi)
Water Purification
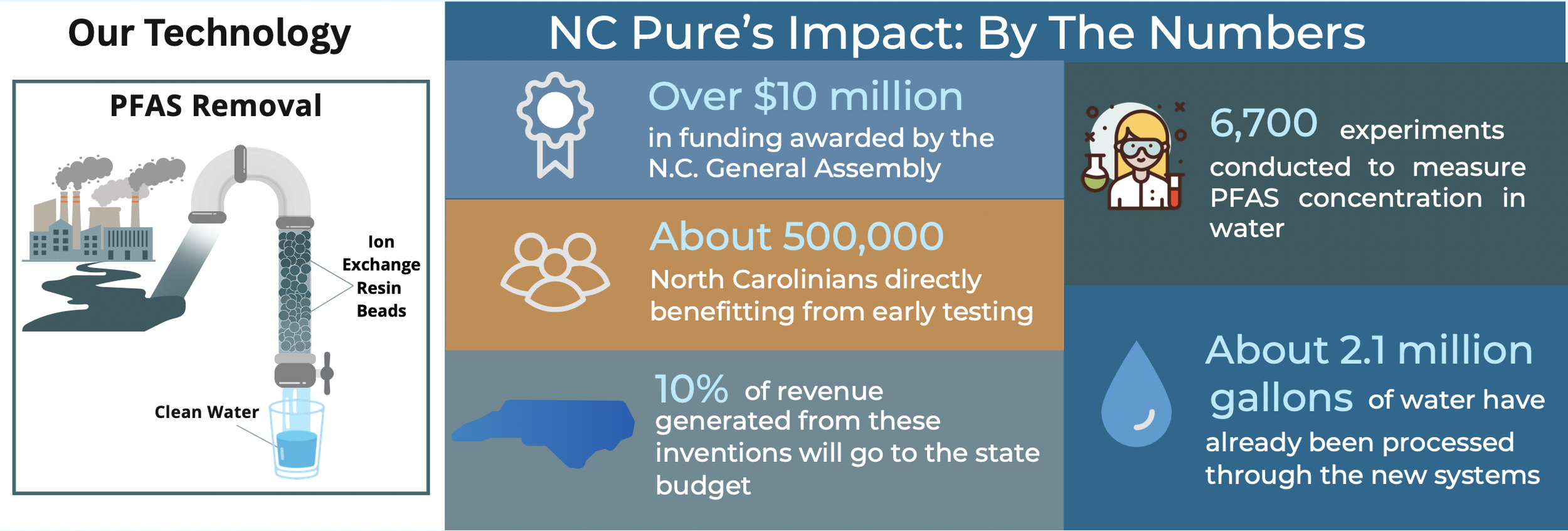
Per- and polyfluoroalkyl substances (PFAS) are small molecules used widely in industrial processes, consumer products, and fire-fighting foams. They contaminate waters worldwide and are associated with adverse human health effects, necessitating effective remediation strategies. Motivated by the limitations of current technologies, we identified design strategies that adsorb PFAS selectively onto granular resins compared to organic matter. Through the NC Pure Program, sponsored by the State of North Carolina, we identified a library of materials, scaled up new sorbents to multi kilogram scale, and evaluated them in pilot studies at municipal drinking water and wastewater utilities in North Carolina. The materials show advantages in the removal of short chain PFAS compared to state-of-the-art ion exchange resins in conditions that mimic full scale implementation of flow-through packed beds.
Representative Publications:
A. Hesterberg Butzlaff, B. Mezgebe, A. Collins, Z. Lin, D. Lassalle-Vega, I. M. Harmody, O. Coronell, F. A. Leibfarth, W. R. Dichtel, M. Nadagouda & M. Ateia, Comparitive Evaluation of PFAS-selective Adsorbents in Hard-to-Treat Residual Waste Streams. Chem. Eng. J. 2025, 511, 161983. (doi)
I. M. Manning, N. G. Chew, H. Macdonald, K. E. Miller, M. J. Strynar, O. Coronell*, & F. A. Leibfarth, Hydrolytically stable Ionic Fluorogels for high-performance PFAS remediation from natural water. Angew. Chem. Int. Ed. 2022, 61, e202208150. (doi)
E. Kumarasamy, I. M. Manning, L. B. Collins, O. Coronell*, & F. A. Leibfarth. Ionic Fluorogels for Remediation of Per- and Polyfluorinated Alkyl Substances from Water. ACS Central Science, 2020, 6, 487-492. (doi)